← Back to periodic table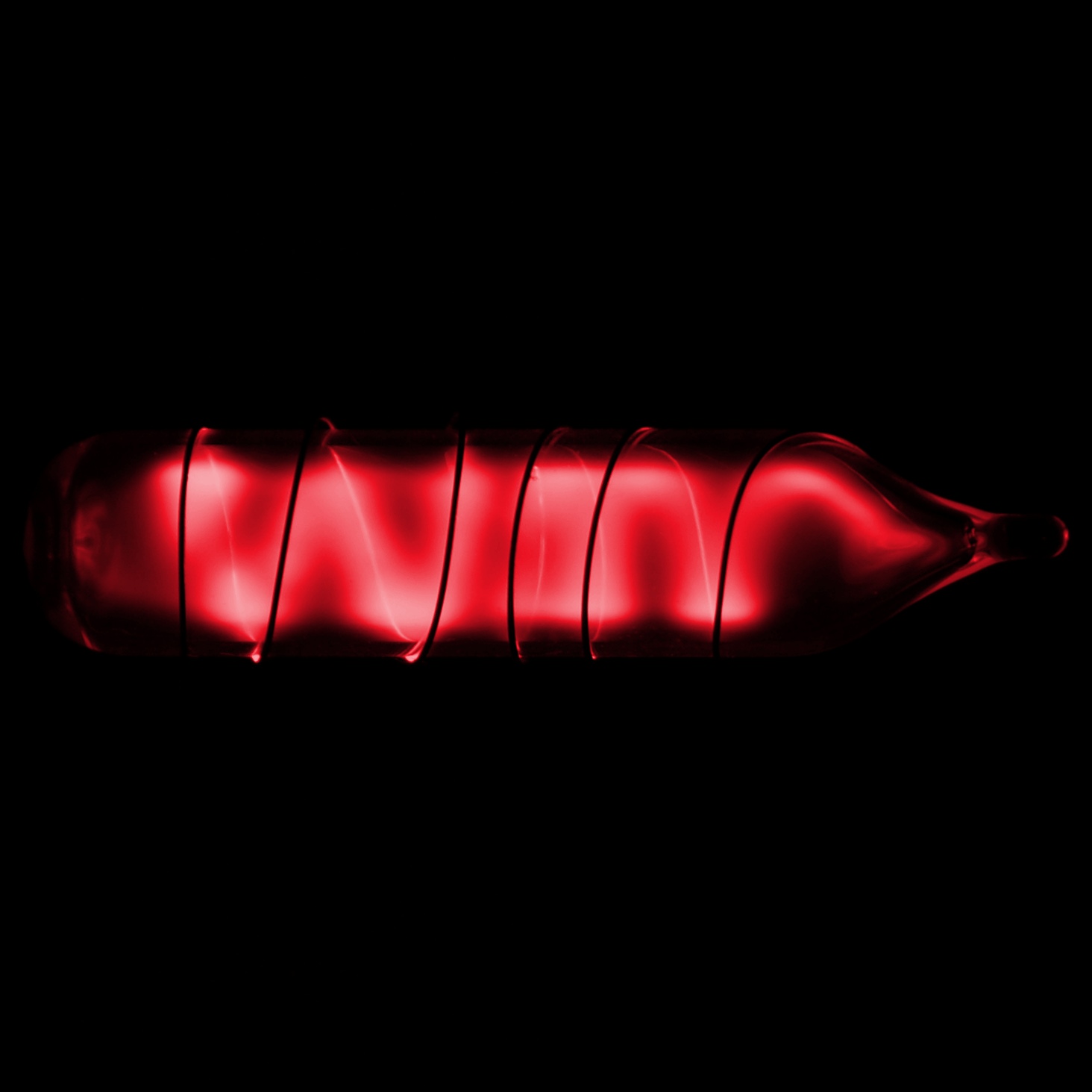
Radon (Rn)
Atomic number: 86 · Category: noble gas · Group 18 · Period 6 · Phase: Gas
Radon is a chemical element with symbol Rn and atomic number 86. It is a radioactive, colorless, odorless, tasteless noble gas, occurring naturally as a decay product of radium. Its most stable isotope, 222Rn, has a half-life of 3.8 days.
Atomic mass: 222
Density: 9.73
Melting point: 202 K
Boiling point: 211.5 K
Appearance: colorless gas, occasionally glows green or red in discharge tubes
Source: Wikipedia
Block: p
CPK color:#428296
Electronic structure
Electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6
Configuration (semantic): [Xe] 4f14 5d10 6s2 6p6
Shells: 2, 8, 18, 32, 18, 8
Electronegativity (Pauling): 2.2
Electron affinity: -68
Ionization energies: 1037
Periodic position
Group: 18
Period: 6
xpos: 18
ypos: 6
Spectral/Bohr images
Image

3D Model


