← Back to periodic table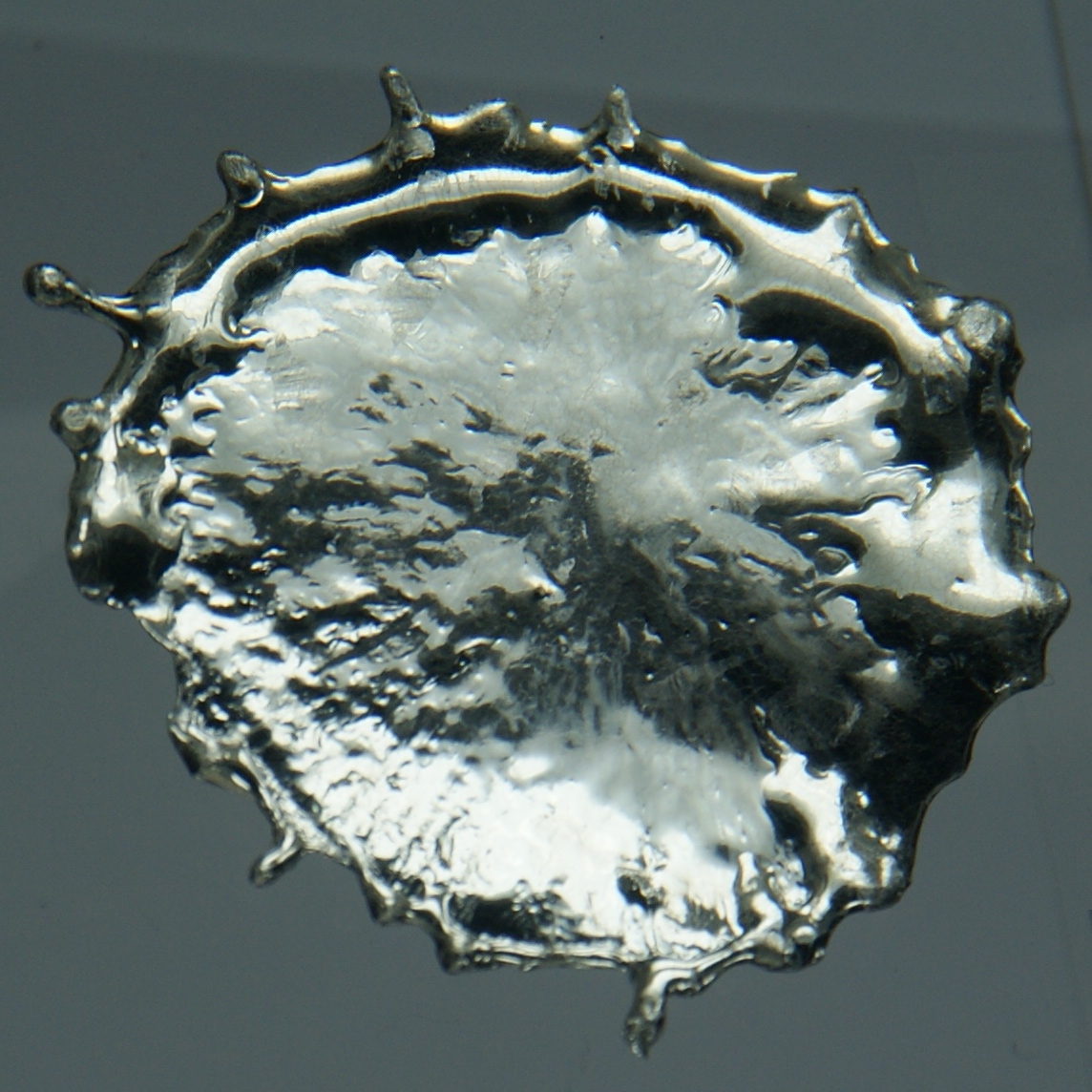
Tin (Sn)
Atomic number: 50 · Category: post-transition metal · Group 14 · Period 5 · Phase: Solid
Tin is a chemical element with the symbol Sn (for Latin:stannum) and atomic number 50. It is a main group metal in group 14 of the periodic table. Tin shows a chemical similarity to both neighboring group-14 elements, germanium and lead, and has two possible oxidation states, +2 and the slightly more stable +4.
Atomic mass: 118.7107
Density: 7.365
Melting point: 505.08 K
Boiling point: 2875 K
Appearance: silvery-white (beta, β) or gray (alpha, α)
Source: Wikipedia
Block: p
Molar heat: 27.112
CPK color:#668080
Electronic structure
Electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p2
Configuration (semantic): [Kr] 4d10 5s2 5p2
Shells: 2, 8, 18, 18, 4
Electronegativity (Pauling): 1.96
Electron affinity: 107.2984
Ionization energies: 708.6, 1411.8, 2943, 3930.3, 7456
Periodic position
Group: 14
Period: 5
xpos: 14
ypos: 5
Spectral/Bohr images
Image

3D Model

